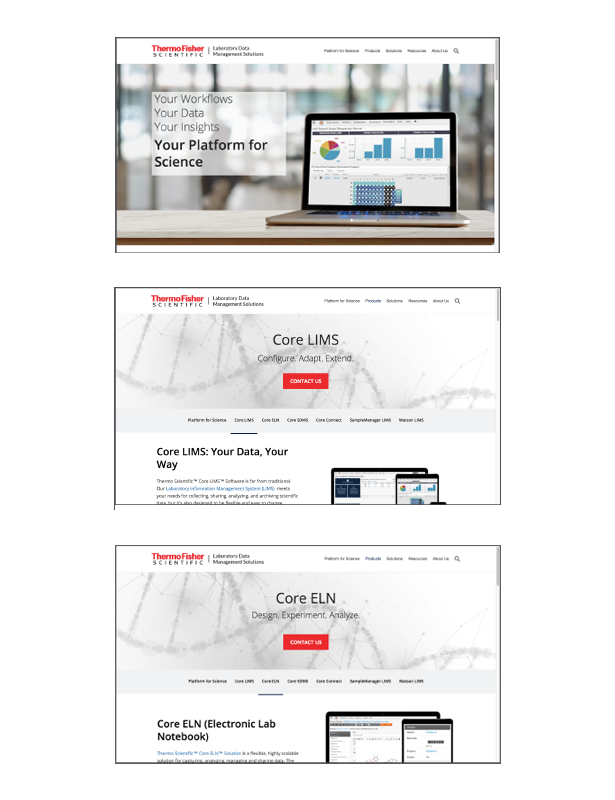
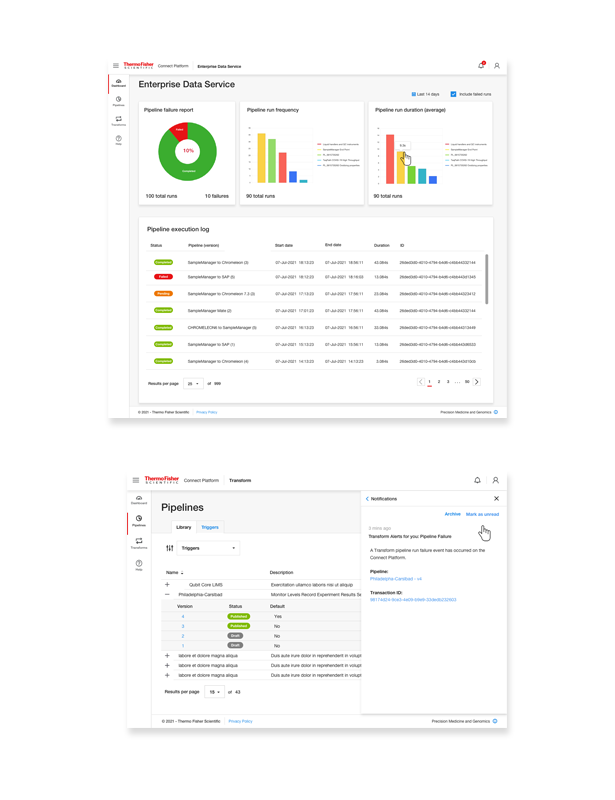
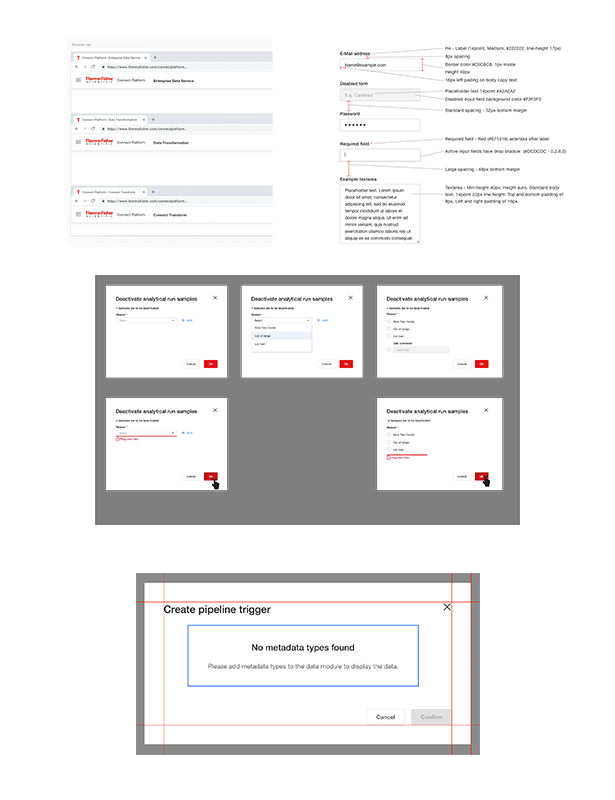
Connect Transform with data integrations
Our client, a leading pharmaceutical company, was struggling with disparate software systems across different departments. Scientists were using multiple tools for research, data analysis, and reporting, which led to siloed data, inefficiencies, and delays in research cycles. With no unified platform, data quality suffered, cycle times were long, and decision-making processes were often delayed due to the lack of real-time insights.>Design For Enterprise Platform Experiences
The goal was to implement Connect Platform Enterprise Edition, integrating their existing software tools, creating a scalable system that would streamline research workflows and improve data-driven decision-making across the organization.
The solution was primarily aimed at scientists, research teams, and system administrators within the organization. Each group had different software needs but required a unified, efficient system that supported their individual workflows while ensuring the integrity of data and the seamless integration of various tools.
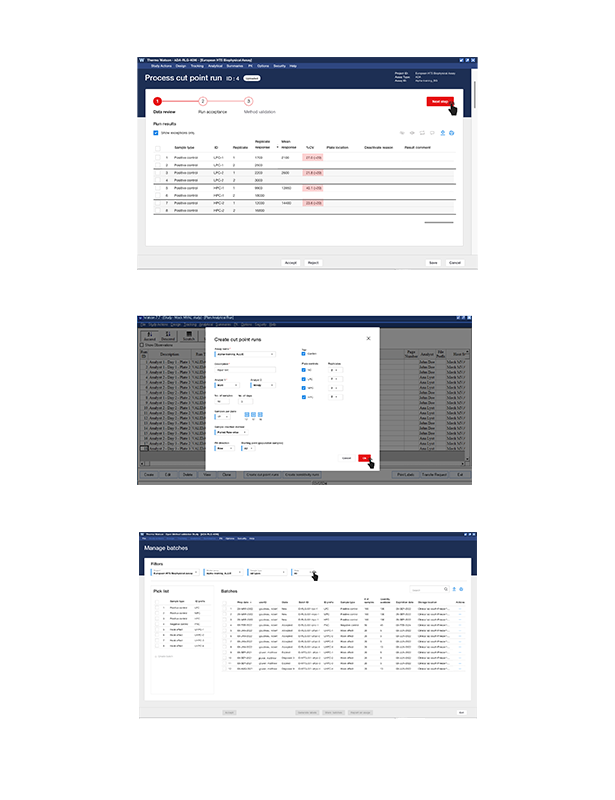
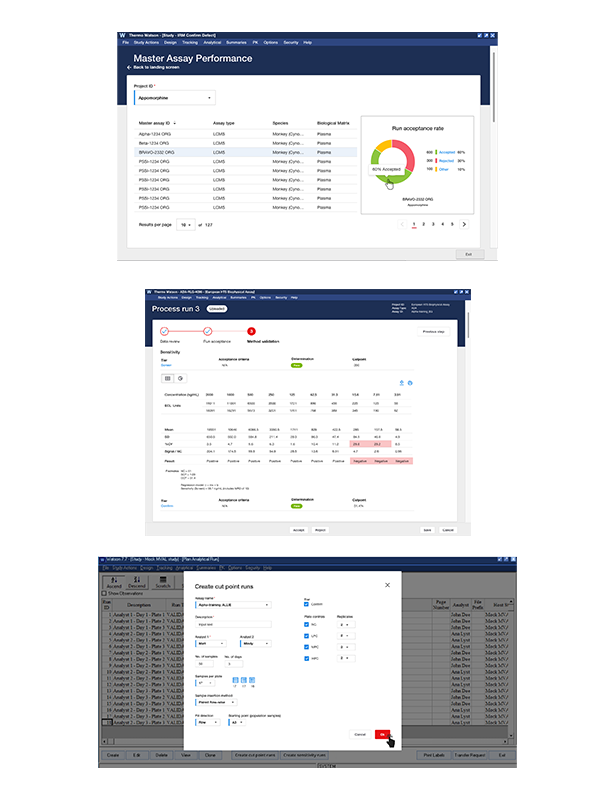
Watson LIMS Immune Response Module
A bioanalytical lab specializing in immunogenicity testing for biopharmaceuticals was struggling to meet the evolving regulatory requirements for ADA (Anti-drug Antibody) validation. Their existing software lacked the tools necessary to effectively develop, validate, and report on ADA assays, leading to inefficiencies, inconsistent data, and potential compliance risks.>Modernize Product thru Digital Transformation
The goal was to modernize the lab’s bioanalytical software to support the complex requirements of ADA method development, validation, and reporting, ensuring full compliance with the new harmonization guidelines.
This project was aimed at bioanalytical scientists, regulatory teams, and lab managers who needed a robust software solution to design, analyze, and report on ADA assays with full compliance to industry standards. The software needed to streamline processes while ensuring data integrity and alignment with regulatory expectations.
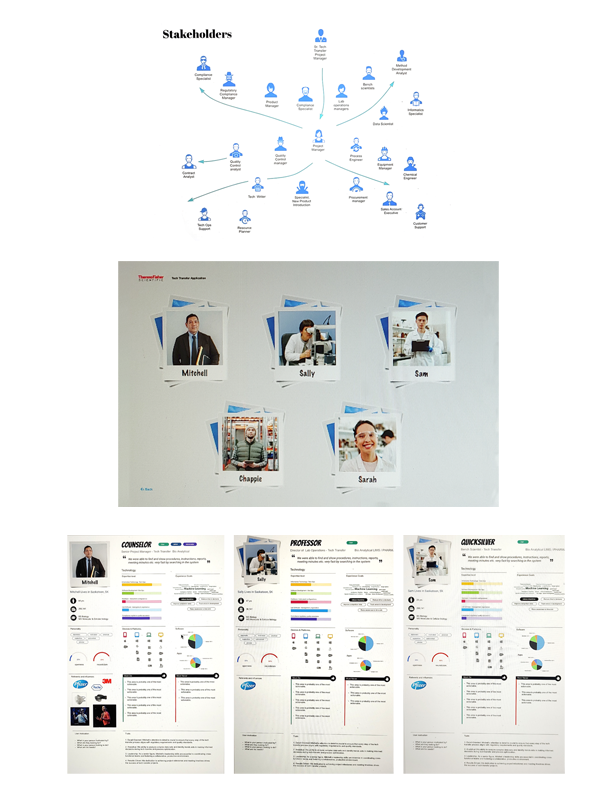
Tech Transfer - New Product Introduction
Our client, a pharmaceutical company, faced a major challenge when one of their key manufacturing plants was unexpectedly slated for closure. This plant was responsible for producing a new small molecule drug that was in the late stages of development and poised for market launch. Without swift action, the company risked a significant delay in bringing the drug to market, potentially losing market share and revenue.>Validation from Prototypes and User Testing
To mitigate this risk, the client needed to transfer the drug’s manufacturing process to another facility and accelerate production to meet the original market timeline. The goal was to execute the tech transfer process efficiently and leverage new digital applications to streamline production and compliance, allowing the drug to be launched six months earlier than initially expected.
This project was critical for the company’s manufacturing team, process engineers, regulatory affairs, and quality assurance specialists, as well as external stakeholders like contract manufacturing organizations (CMOs). The objective was to ensure all teams were aligned during the tech transfer to maintain quality, compliance, and speed.